
Above 49 days after mating


2010--Remaining 4 eggs at 4 weeks. Have increased some more in size,

2010--Eggs at 63 days. Notice the difference in shapes than at 4 weeks above.
2010--First Simus emerging from egg at 68 days.

The final baby emerges on 10/5/10


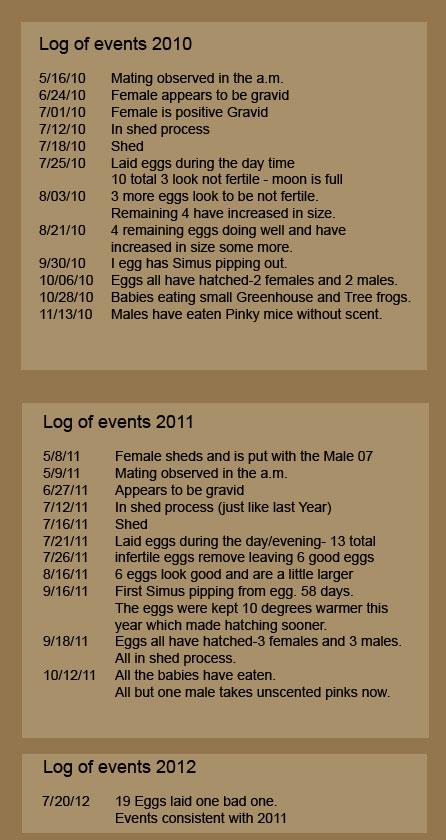
First Simus pipping from egg 9/16/2011
HETERODON SIMUS (Southern Hog-nosed Snake). REPRO- DUCTION and NESTING. Heterodon simus is a highly fossorial species associated with xeric sandhill habitats within the declin- ing Longleaf Pine (Pinus palustris) ecosystem of the southeast- ern USA. Its relative rarity and secretive nature have confounded documentation of certain basic natural history information. Natural nests have long gone unreported (Jensen et al. 2008. Amphibians and Reptiles of Georgia. The University of Georgia Press, Athens, Georgia. 575 pp.), with reproductive information being limited to observations of copulating pairs and egg clutch- es that were either oviducal or deposited by captive females (e.g., Palmer and Braswell 1995. Reptiles of North Carolina. University of North Carolina Press, Chapel Hill, North Carolina. 412 pp.; Beane et al. 2014. Copeia 2014:168–175). Herein, we describe an apparent failed natural nest of H. simus.
On 23 May 2020, one of us (DCS) found a gravid female H. simus (ca. 425 mm SVL, 485 mm total length, 153 g) crossing a sand road (ca. 15 km NW of Wagram, Scotland County, North Carolina, USA). She was confirmed gravid by a radiograph and surgically implanted with a radio transmitter (Holohil SB-2T, Holohil Systems, Ltd., Carp, Ontario) on 26 May 2020, released on 31 May 2020 at point of capture, and monitored frequently (located on 52 of the 71 dates between 1 June and 10 August). For the entire monitoring period, the snake utilized relatively open sandhills dominated by P. palustris, Quercus incana (Bluejack Oak), Q. laevis (Turkey Oak), and Aristida stricta (Carolina Wiregrass), and on a two- to three-year prescribed burn rotation. From 6 June to 14 July, she utilized a single burrow of her own construction (Fig. 1) and was found underground in that refugium on 25 dates, and on the surface only twice, during that period. On both occasions observed on the surface (30 June and 1 July) she was basking ca. 5 m from the burrow and still appeared noticeably gravid. Between 15 and 27 July, she used four additional burrows, all within 61 m of the most-used burrow, and on 27 July she was observed active on the surface and no longer appeared gravid. On 10 August 2020, she was found dead and partially consumed by a predator. The condition of the remains suggested a raptor.
We suspected eggs had been deposited in the burrow the snake used for the longest period (6 June–14 July). From 25 August–5 October, a hardware cloth nest protector was placed over that burrow, as well as over each of the four additional burrows known to have been used between 6 June and 1 August. Each burrow was monitored daily. No indications of hatching or emergence were noted. On 31 October 2020, we carefully excavated the most-utilized burrow. A nest chamber was located off a side tunnel ca. 61 cm lateral distance from the burrow entrance. The chamber measured ca. 7 × 7 cm and was situated at the interface of the topsoil and roots of a tussock of A. stricta and the underlying layer of sandy soil (Fig. 2). The bottom of the chamber was ca. 25 cm below the surface. The chamber contained six non-adherent eggshells in an advanced state of degradation; none appeared to have hatched (Fig. 3). Although it was difficult to determine the precise number of ova visible in the radiograph, it was clearly more than six and may have been greater than 13. It is possible that some eggs were either reabsorbed or never developed fully, were infertile or otherwise deteriorated completely before we discovered the nest chamber, were deposited in another location, or were consumed by an underground predator. JCB and SJH found the shed skin of an adult Cemophora coccinea copei (Northern Scarlet Snake), a known reptile egg predator, within ca. 1 m of the nest burrow on 3 July. The remains of the female and eggshells are deposited in the herpetology collection of the North Carolina State Museum of Natural Sciences (NCSM 104650).
We thank J. B. Minter, Emma Houck, and Kelly Tardiff for performing the transmitter implant and providing the radiograph, Thomas J. Thorp for providing the transmitter; and Zachary and Dillon Smith for assistance with nest excavation. Endangered species permits were provided by the North Carolina Wildlife Resources Commission (20-ES00019, 20-ES00245, 20- SC00218). Support was provided by Project Simus, an initiative of the North Carolina Herpetological Society.
JEFFREY C. BEANE, North Carolina State Museum of Natural Sciences Research Laboratory, MSC #1626, Raleigh, North Carolina 27699-1626 USA (e-mail: jeff.beane@naturalsciences.org); MICHAEL D. MARTIN North Carolina Wildlife Resources Commission, 2995 US-1, Hoffman, North Carolina 28347, USA (e-mail: michael.martin@ncwildlife.org); DUSTIN C. SMITH, 1284 Briarcliff Drive, Asheboro, North Carolina 27205, USA (e-mail chondro_13@yahoo.com); STEPHANIE J. HORTON, North Carolina Natu- ral Heritage Program, Nature Research Center, 121 W. Jones Street, Raleigh, North Carolina 27699-1651, USA (e-mail: stephanie.horton@ncdcr.gov).





H Simus 70 days in egg at 74-76 degrees.

2013 breeding activity

2012 Red phase pair. All have grown quite nicely. 02/04/2013

Out of 12 good eggs this year I had 6 red phase and 6 normal H. Simus.
Here is one of the red phase.

2012 babies a few days after hatching. Note some are still shedding.
9/24/2012 Simus pipping from egg 65 days

Simus babies 10/28/10

